New Elements Added to the Periodic Table
Cool Chemistry Creations: With elements 113, 115, 117 and 118 discovered by man-made means, the seventh row of the periodic table is now completed.
Jan 3, 2016
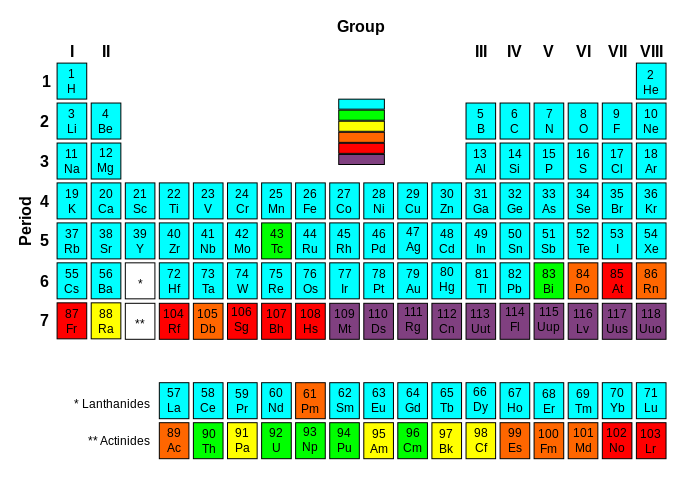
Recently, evidence proved the existence of four new elements of the periodic table. Elements with atomic numbers 113, 115, 117, and 118 were all discovered, completing the seventh period, or row, of the periodic table of elements. The existences of these elements were verified by the International Union of Pure and Applied Chemistry (IUPAC) on Dec. 30, 2015.
Elements 115, 117, and 118 were discovered by a team of American and Russian researchers. The discovery of element 113 was credited to a Japanese team at the Riken Institute. The teams responsible for these discoveries have been invited to give permanent names and chemical symbols to the elements. For the time being, elements 113, 115, 117, and 118 are Ununtrium, Ununpentium, Ununseptium, and Ununoctium respectively. Permanent names of elements can originate from mythological concepts, minerals, places, properties or scientists. In order to make these elements, researchers had to smash together two smaller elements to create an element with a large atomic number.
“That’s how you discover synthetic elements. You fuse them. You actually create a bigger nucleus by fusing them {two smaller elements} together,” Chemistry Teacher Mr. Cartas said.
Even so, the elements were so chemically unstable that they existed only for a fraction of a second before decaying and disappearing. For example, element 113, which was created by smashing bismuth with zinc ions, decays in less than a thousandth of a second, as reported by its Japanese discoverers. The reason these elements are incredibly unstable has to do with the amount of protons and electrons they have. Because they have an immense amount of both a negative charge, from electrons, and a positive charge, from protons, the opposite charges repel too strongly for the atom to maintain stability and thus for it to exist for more than a fraction of a second. Not only was it challenging to study an element that only lasts for less than a thousandth of a second, but attaining enough of the two elements that were smashed together to form one of the new elements was incredibly difficult. It took two years for researchers to collect only 13 milligrams of Berkelium and create element 117 because of its rarity.
The existence of the four new elements has inspired new discoveries for elements of even greater atomic numbers. By continuing research for new and heavier elements, it may be possible to eventually find an element both stable and heavy enough to have practical applications.
“This new discovery, despite its limitation, is a huge step for science in the sense that it allows us to have a better understanding of our world, which is a large purpose of science in general,” junior Mariam Ahumada said.